What is the MED Marine Approval?
The MED Mark of Conformity confirms that a product or piece of equipment is approved for use on board EU member state ships and ships of countries which have agreed to apply the Marine Equipment Directive 96/98/EC. This is sometimes referred to as the ‘wheelmark’ or ‘wheel mark’ and also known as M.E.D/MED approval or certification. It provides confirmation that the equipment or product is suitable for marine industry use.
This certification is also valid in the U.S. based on the Mutual Recognition Agreement (MRA) for testing and approvals according to the 96/98/EC Directive. In short, MED products listed in the agreement are to be recognized as complying with U.S. standards without any further testing.
All approved and authorized products are stored in the MarED database which contains information about over 35,000 authorized devices. Products are tested by an independent third party, such as Germanischer Lloyd, for use in the marine environment before being granted an approval certificate.
Did you know?
As stated in the MED-regulations:
Should the specified regulations or standards be amended during the validity of this certificate, the product is to be re-approved before being placed on board a vessel to which the ammended regulations or standards apply.
This means for the user that the MED certificate is invalid if the applied standards and regulations change. In this case, the products can no longer be used in shipbuilding. The certification of the products must be requested again.
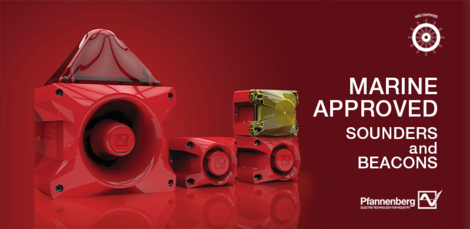
Our MED Certified visual and audible devices
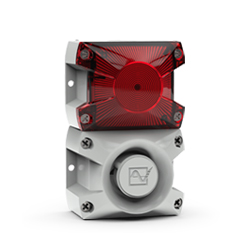
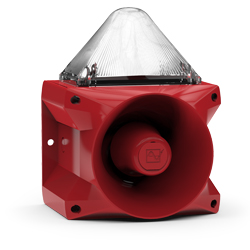
Pfannenberg offers an extensive range of products and solutions designed specifically to withstand the rigors of the marine industry. Pfannenberg PA sounders and PA X combined sounders/beacons are MED certified by Germanischer Lloyd (a Notified Body under the terms of the MED).
Germanischer Lloyd has confirmed that Pfannenberg’s PA sounders and PA X combined sounders/beacons meet the requirements of the directive.